NEWS
September 30th – ICEMVS stabilisation period comes to an end.
1st. October 2019
Stabilisation period granted to end-users of the Icelandic Medicines Verification system (ICEMVS) has come to an end as of September 30th, 2019. End-users in Iceland are now required to record, investigate and potentially report alerts received from ICEMVS and the European Medicines Verification System (EMVS).
In case of an alert, the end-user is advised to:
-
- Record the event in line with users operating procedure on deviations.
- User should exclude any technical scanning errors or other local software issues that might have triggered the alert. User should also check for known pack or batch issues caused by EMVO Onboarding partner (OBP) and registered with ICEMVO.
- ICEMVO can provide support to rule out technical issues and facilitate flow of information between end-user and OBP.
- End-user should take a picture of the pack and 2D-barcode, put it aside and evaluate further need for investigation (e.g. has the seal been tampered with, does the pack look suspicious?).
- If technical errors have been excluded and information about the pack-alert is not received from OBP with-in two working days, the respective wholesaler shall be notified. Information to be provided to the wholesaler includes product code (GTIN, PC number), serial number (SN), expiry date and Unique Alert ID. It´s very important not to initiate a return procedure of the product without first consulting the respective wholesaler.
- In the case that a possible falsification can not be ruled out after consulting wholesaler and MAH/OBP representative in Iceland, a formal notification of a suspected falsified product should be sent to the Icelandic Medicines Agency (IMA).
ICEMVO 2019 Annual Fee
04.03.2019
The ICEMVO annual flat fee is charged per market authorization holder to cover, inter alia, the annual costs of the operation and further development of the ICEMVS, costs inherited from the European Medicines Verification Organization and all necessary and legally compulsory activities of the ICEMVO. The level of the annual flat fee contribution will be based on actual cost of running the ICEMVO short and long term.
The amount of the yearly flat fee (IT-cost and operational cost of ICEMVO divided by number of MAH’s) may fluctuate from time to time.
ICEMVO 2019 Annual Fee has been decided by ICEMVO Board of Directors. It will be EUR 4.900 for MAH with IDM sales turnover above 10 m ISK (80k EUR) and EUR 1.500 for MAH with IDM sales turnover below 10 m ISK (80k EUR).
For any further information please contact ICEMVO via email info@lyfjaaudkenni.is
Verification of medicines in Iceland from 9th February 2019 – initial stabilisation period or ‘use and learn’ phase
08.02.2019
The following national approach to the initial operation of the medicines verification system in Iceland has been announced today.
The Icelandic Medicines Agency has been working closely with the ICEMVO to monitor progress in the implementation of the new safety feature requirements across all sectors. Notwithstanding the significant work undertaken to date and given the complexities associated with setting up the medicines verification system impacting all stakeholders in the medicines supply chain across Europe, it is anticipated that the initial period of operation will identify issues as the new system comes into effect Europe-wide.
Following discussions at a national and European level with stakeholder representatives, it has been decided to adopt a pragmatic approach to the implementation of the Delegated Regulation (and the associated statutory instrument) after the go live date, to ensure the continuity of safe supply of medicines to patients while all parties gain a better understanding of the new system. This means that:
- All medicinal products released by MAHs for the Icelandic Market after Feb 9th should bear the safety features as required i.e. a tamper proof seal and 2D barcode
- During the initial period of operational stabilisation, the system will be considered to be in ‘use and learn’ phase. Therefore wholesalers, pharmacies and hospitals should scan medicines bearing the safety features and if an alert or any other unexpected message is flagged, should continue to supply packs to patients in accordance with their existing procedures, unless they have overriding concerns that a falsified medicine is involved.
- All alerts generated by FMD system in pharmacies, wholesalers and hospitals, upon scanning a pack during this ‘use and learn’ phase will be forwarded by the system to the ICEMVO, the IMA and the pharmaceutical companies so that they can be investigated and monitored.
- Notwithstanding the above, if a pharmacist or wholesaler has reason to believe that packaging has been interfered with, based on their examination of the anti-tampering device on the pack, they must report their concern to the IMA (as a suspected quality defect via the usual reporting mechanisms) and not supply the pack.
During this period, the alerts generated will be analysed by the IMA and ICEMVO to determine why they are occurring and develop appropriate protocols for their resolution. Manufacturers (and marketing authorisation holders (MAHs)) will also be involved in the investigation of alerts generated against their products. The stabilisation period will be from February 9th to September 30th. This approach will ensure that medicines continue to be provided to patients without delay and also ensure that all stakeholders, manufacturers, wholesalers, pharmacists and other healthcare professionals work to build confidence in operating the new safety system.
Formal notification of this agreed national approach has been issued today to pharmacists, wholesalers, MAHs and manufacturers by the IMA (only in Icelandic). ICEMVO is very appreciative of the careful consideration given to the matter by the authorities in Iceland and we will continue to work closely with them over the coming months to maximise the learnings about the system during this ‘use and learn’ period.
ICEMVO activities regarding alerts
All communications with ICEMVO about alerts should be emailed to alerts@lyfjaaudkenni.is. MAHs registered with us are asked to nominate a contact point for ICEMVO to liaise with when we have queries about alerts relating to their products.
New EMVO system to provide information on status of EU Hub and national system.
The European Medicines Verification System Information (EVI) has been launched by EMVO to provide information on downtimes and known issues with the EU Hub and all the national systems (including the Icelandic system). You can consult the EVI and subscribe for updates by clicking here.
Contacts in ICEMVO
| Type of query | Email address |
| Queries re MAH fees, MAH onboarding, invoices, MAH agreements, etc. | info@lyfjaaudkenni.is |
| Queries relating to end-user (pharmacy, hospital, wholesaler) registration with ICEMVO | info@lyfjaaudkenni.is |
| Queries relating to alerts | alerts@lyfjaaudkenni.is |
| All other queries*
*Queries about EMVO onboarding and uploading pack data to EU Hub, should be directed to EMVO Helpdesk (Tel.: +372 611 90 44, e-mail: helpdesk@emvo-medicines.eu) |
info@lyfjaaudkenni.is |
Announcement – Icelandic Medicines Verification System Goes Live
14.05.18
We are delighted to announce that on May 14th, the Icelandic Medicines Verification System (ICEMVS) was connected to the European Hub live production environment.
Achieving this important milestone was made possible with successful collaboration with colleagues from other NMVO’s working with Solidsoft Reply, especially ZAPAZ in Slovenia, with strong support from the EMVO team and finally ICEMVO Board members, who have actively engaged in this project during the last two years.
Commencing in the coming weeks, we are starting the next phase of our project with participants who are ready to onboard to the EU Hub and with controlled connection of end-users to the national system. This will provide enough time to all participants in the pharmaceutical supply chain to onboard, test, train and prepare themselves in time.
We would encourage manufacturers and OBP´s to start uploading product and pack data to the EU Hub destined for the Icelandic market, including multimarket packs with Denmark and Sweden – ICEMVO is now connected.
Finally, thank you to the many MAH´s who have already completed ICEMVO´s MAH onboarding procedure. If you have not yet registered, details of how to do this are available at http://lyfjaaudkenni.is/en/ma-holders/ .
For further information about ICEMVO, please visit our website http://lyfjaaudkenni.is/en/icemvo/ or get in touch with us by email (info@lyfjaaudkenni.is) at any time.
June is last call to start on-boarding the EU-hub
EMVO is making all On-Boarding Partners (OBP) aware that the end of June 2018 should be considered as the very last opportunity to On-board timely.
An On-boarding Partner is EMVO’s designation of a Marketing Authorisation Holder (MAH) who uploads serialized data.
EMVO would like to make all On-boarding Partners and future On-boarding Partners aware that the end of June 2018 should be considered as the very last opportunity to On-board timely. Any late on-boarding after that date may entail a risk to the company’s business.
The OBP has to take into account that information and expertise from various departments of its company are needed to complete the contractual and technical on-boarding on time. EMVO’s pledge is to ensure an effective level of service towards all OBPs and to allow sufficient time for a successful on-boarding. On the other hand, the OBP should be aware that the on-boarding process may take some time, beyond the control of EMVO
There are more than 2,000 OBPs in Europe, and EMVO is concerned that that many OBPs will initiate their connection to the EU Hub late. There are still too many OBPs who are not connected to the EU Hub, and that is why EMVO makes them aware that EMVO can’t guarantee those who initiate on-boarding after June 2018 that they will be on-boarded in time to upload serialized data before February 9th 2019.
Read the letter from EMVO:
Letter of Announcement – Last Opportunity for a Timely On-boarding
More information on boarding from EMVO:
ICEMVO takes part in a successful joint UAT of the blueprint verification system
24/01/2018
We are delighted to inform that the User Acceptance Test (UAT) of the Solidsoft Reply national blueprint system took place on schedule in Slovenia this week, led by Slovenian NMVO (ZAPAZ), and that the system has passed the UAT. This is a key milestone in the development of the national verification systems.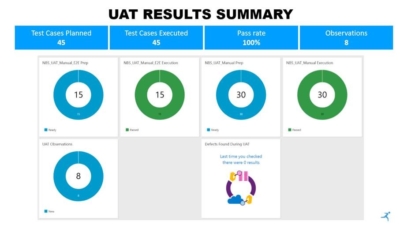
Full end-to-end testing of the system took place using pack data uploaded by J&J and Krka to the EU Hub and with the 2D data matrix codes scanned using Slovenian pharmacy and wholesaler IT systems. The testing took place in the EU Hub and Solidsoft Reply national blueprint system quality environments which are identical to the relevant live production environments.
Event was witnessed by representatives from all involved NMVOs, EMVO and Slovenian stakeholders.
Thanks to involved NMVO community for perfect collaboration and Solidsoft Reply for the professional approach! NMVO´s that participated in this event include Iceland, Denmark, Sweden, Ireland, Lithuania, Czech, Bulgaria, Slovenia and Croatia.

